Battery is the new Oil!
Electric Vehicles (EVs) have been around in various shapes and forms even before the advent of Internal Combustion (IC) vehicles. Nearly, 30% of all vehicles on the road were electric cars in that era, but the availability of cheap gasoline and inexpensive cars pushed research into better battery technology to the back burner.
It was not until the 1970s that interest in EVs got renewed, and the development of higher energy density batteries came to the research forefront. Recently, the battery technology has made mass-production electric cars a reality, thanks to the technology advancement. Still, EVs cannot just be made by replacing the engine and fuel tank by electric motors and electric batteries. It is an evolving technology driven to new heights by companies like Tesla, Nissan et al.
No doubt, the heart of an EV is its battery. Unlike the batteries in IC cars, which primarily serve to start the engine and run accessories like the lights, wipers, radio and/or ACs, the battery in an EV runs everything.
Importantly, it runs the electric motor, so it needs to be powerful and long-lasting enough to take drivers where they need to go with a minimum of recharging. Until recently, no reliable, mass-producible batteries were manufactured that could make electric cars competitive with IC cars. But, today EVs have not only become feasible, but they are now rolling off the assembly lines of major automobile manufacturers.
Historical Overview
Ever since the discovery of electricity, humans have sought to have simple yet portable source of current in form of a battery. Though, the challenge was that it had to balance; power, weight, cost, and some other factors. Scientists have worked for over a century to get to today’s level of battery efficiency which included many trade-offs. Highlighted below are some of the important milestones of this journey.
Major Milestones in Battery Development

Evolution of Batteries over the years for EV application
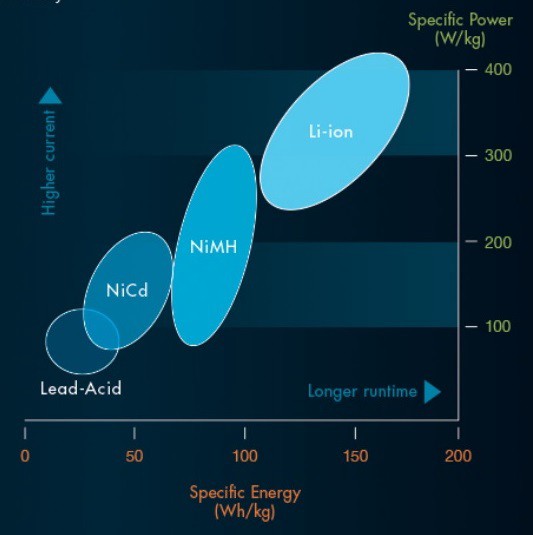
Several battery chemistries have been introduced including improved lead–acid, nickel–cadmium, nickel–zinc, NiMH, zinc–air, sodium–sulfur, sodium–metal chloride, Li-ion batteries in the EV segments.
The experimentation with battery chemistries is due to fact that, for EVs there are several factors that could affect battery choice, including cost. However, two factors that determine the fit and use of rechargeable batteries specifically are:
- Specific Energy — the amount of energy a battery holds in total.
- Specific Power — the amount of current a battery can supply for a given use.
It is depicted in the info-graphic below:
Reviewed below are some of the battery technologies being used in the EVs and the state-of-art, emerging technological development in the field.
Lead-acid
There are 2 types of lead-acid batteries, automobile engine starter batteries, and deep cycle batteries. Automobile alternators are designed to provide starter batteries high charge rates for fast charges, while deep cycle batteries used for electric vehicles like golf carts or e-rickshaws. Historically, most EVs have used lead-acid batteries due to their mature technology, high availability, and low cost.
Lead-acid batteries in EV applications end up being a significant (25–50%) portion of the final vehicle mass. Like all batteries, they have significantly lower energy density than fossil fuels — in this case, 30–40 Wh/kg. However, due to lighter drive-train in EV, higher weight of batteries is partly compensated. Still, with the best batteries EVs of a range comparable to IC vehicles tend to lead to higher weight. Recent advances in battery efficiency, capacity, materials, safety, toxicity and durability are likely to allow these superior characteristics to be applied in car-sized EVs.
Lead-acid batteries powered such early-modern EVs as the original versions of the EV1 and the RAV4 EV.
Due to further developments in EV application, VRLA batteries emerged. A valve-regulated lead-acid battery (VRLA battery) also called sealed lead-acid (SLA) battery. Another type of VRLA battery is the Gel and Absorbent Glass Mat (AGM) types of VRLA which can be mounted in any orientation with minimal maintenance.

In India, most EVs are either three wheeled rickshaws or two wheeler. Both the segments are highly price sensitive.
The fact of the matter is that, in India, lead-acid takes the cake with its definite pricing edge. The initial cost of a lead-acid battery pack is much cheaper than its equitable lithium-ion battery (LIB) pack.
Although, the advantages of LIB may justify its price on many levels, the Indian consumer is wary of having to shell out this additional investment in the aforesaid segments.
Specially developed VRLA batteries for EVs application are widely available and are considered as a competitively priced product, the cost of initial purchase is affordable to an EV owner and in the long run, replacements are also favorable in terms of their price. Also, the lack of lithium-in India and the fact that lithium-ion technology for electric cars is a whole new territory and can’t be integrated into current manufacturing regimes.
NiCad battery
The nickel–cadmium batteries (NiCad) is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes and were about 50% more energy dense than the lead-acid. NiCad batteries can be made in a wide range of sizes and capacities. Compared with other types of rechargeable cells, they offer good cycle life and performance at low temperatures with a fair capacity but their significant advantage is the ability to deliver practically their full rated capacity at high discharge rates (discharging in one hour or less). However, the materials are more costly than that of the lead–acid battery, and the cells have high self-discharge rates.
NiCad was state of the art batteries all through the 1990’s. They were used in quite a few EVs in France and US. However, the environmental impact of the disposal of the toxic metal cadmium has contributed to waning of this technology for EV applications.
Nickel Metal Hydride
The demise of NiCad batteries for EVs led to development of Nicket-Metal-Hydride (NiMH) batteries which are about 200% as energy-dense as lead-acid. These were first used by Toyota Prius and Honda Insight hybrid electric cars. However, an NiMH electric car battery pack would simply be too heavy to achieve range improvements.
NiMH batteries are now considered a relatively mature technology. While less efficient (60–70%) in charging and discharging than lead-acid, they have an energy density of 40–80 Wh/kg, far higher than lead-acid. When used properly, NiMH batteries can have exceptionally long lives, as has been demonstrated in their use in hybrid cars and surviving NiMH RAV4 EVs that still operate well after 100,000 miles (160,000 km) and over a decade of service.
NiMH battery used in the second generation EV-1 and worked very well. By 2008, more than two million hybrid cars worldwide were manufactured with NiMH batteries.
However, the status of NiMH batteries in EV segment diminished over time due to the increased popularity of lithium-ion batteries.
Li-ion battery
A lithium-ion battery (Li-ion/LIB) is a type of rechargeable battery in which lithium- ions move from the negative electrode to the positive electrode during discharge and back when charging. Li-ion batteries use an intercalated lithium compound as one electrode material, compared to the metallic lithium used in a non-rechargeable lithium battery. The electrolyte, which allows for ionic movement, and the two electrodes are the constituent components of a Li-ion cell.
Lithium batteries were initially developed in the 1970s by using titanium (IV) sulfide and lithium metal as the electrodes. However, titanium disulfide was a poor choice, since it has to be synthesized under completely sealed conditions, also being quite expensive. When exposed to air, titanium disulfide reacts to form hydrogen sulfide compounds which are toxic to most animals.
Batteries with metallic lithium electrodes presented safety issues, as lithium is a highly reactive element; it burns in normal atmospheric conditions because of spontaneous reactions with water and oxygen.
As a result, research moved to develop batteries in which, instead of metallic lithium, only lithium compounds are present, being capable of accepting and releasing lithium ions.
The graphic below shows the currently usable Lithium compounds in LIBs.
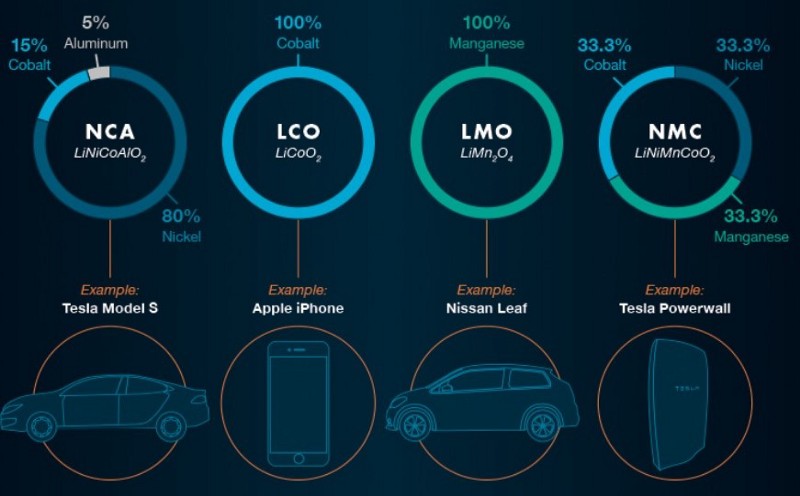
This would only be possible with constant improvement of LIBs based on multiple advances. Some of these are as follows:
Efficient Manufacturing
Tesla has already demonstrated significant advances in battery design and production through its Giga-factory. Some of the other players are going to follow the game by better engineering and manufacturing processes, wider and longer cell design allows more materials packaged into each cell and new battery cooling system allows to fit more cells into battery pack.
Better Cathodes: Cathode optimization by manipulating the relative quantities of cobalt, aluminum, manganese and nickel into LIBs, it has significantly increased the energy density. Research is going on to find the materials that store maximum amount of ions.
Better Anodes: Using silicon anodes instead of current graphite ones can increase battery capacity by ten folds. But, silicon anodes have a problem of bloating in size, thus damaging the anode and finishing battery life. Some new approaches to make silicon anode workable include:
- Encasing silicon in a graphene “cage” to prevent cracking after expansion.
- Using silicon nano-wires, which can better handle the volume change.
- Adding silicon in tiny amounts in current graphite anodes.
Solid-State Lithium-Ion: High performance batteries can be solid state ionic devices. To achieve the higher energy density in lower cost, development of a solid state electrolyte is essential. In 2017, a new solid-state battery has been unveiled, using glass electrolytes and an alkali-metal anode consisting of lithium, sodium. This design could increase energy density, but it still has some problems to resolve, such as ions moving too slowly through the solid electrolyte.
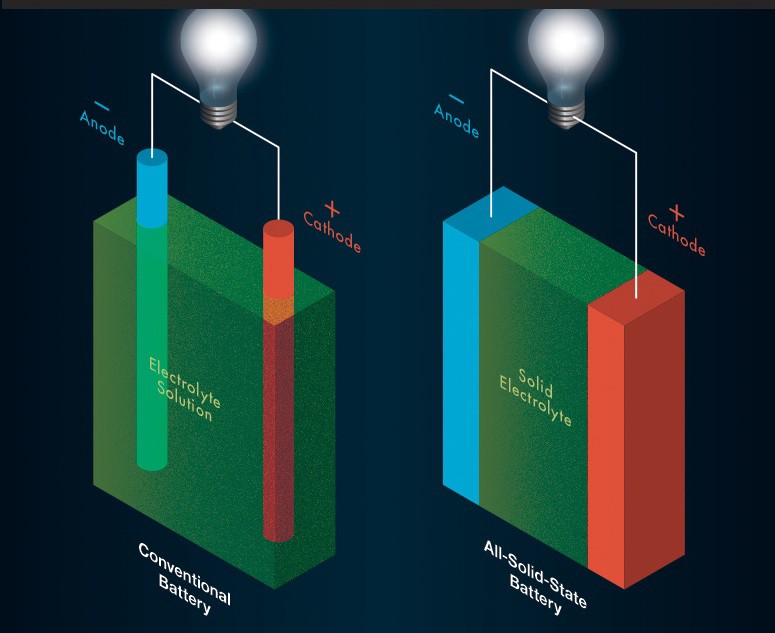
Emerging EV Battery Technologies
Currently, there are several approaches to battery innovations that may lead to making EVs ubiquitous. Certainly, path to commercialization is long, hard and may have pitfalls too. The graphic below summarizes the possible paradigm shift in the EV battery approach.
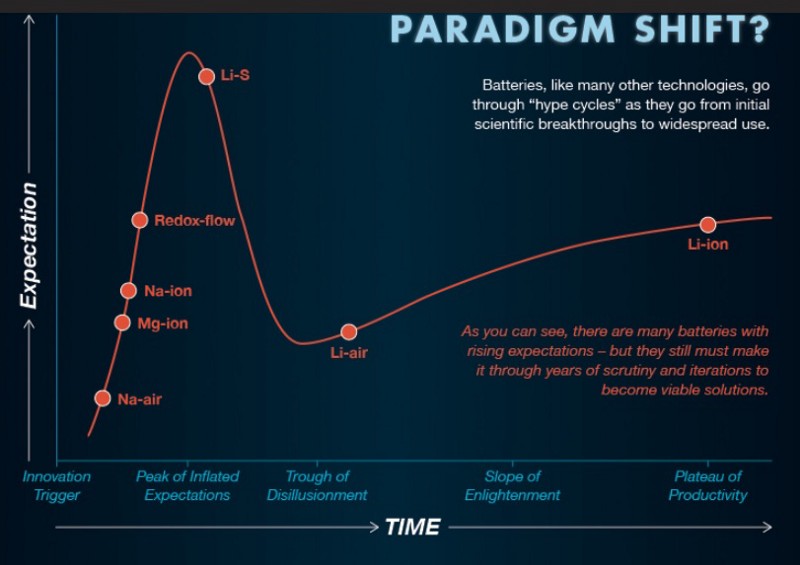
Extending Lithium-ion in other ways
There are various ways in which researchers are exploring use of lithium-ions in other chemistries and methods. Some of these are summarized below:

Metal Air Batteries
Some time back, there was an impetus on using metal air batteries, which subsided as the technology could not emerge and LIBs made major stride. Recently, there has been a renaissance in development of this technology.
Aluminum Air Batteries
A new found focus on Aluminum–air batteries has emerged. The driver being that Al-air chemistry has one of the highest energy densities among all batteries. In the past, high anode cost and efficient byproduct removal was a challenge. An electric vehicle with Al-air battery can possibly achieve up to eight times the range of a LIB with a lower weight.
Al-air batteries are primary cells, i.e., non-rechargeable. The aluminum anode is consumed by reacting with atmospheric oxygen (cathode) immersed in a water-based electrolyte to form hydrated aluminum oxide, the battery will no longer produce electricity. Mechanically, it is possible to resurrect the battery with new aluminum anodes. These could be recycled from aluminum hydroxide that is produced.
Phinergy, an Israeli company, has developed innovative aluminum-air battery that is capable of providing energy to power an electric vehicle (EV) for up to 1000 miles at a time albeit, with stops every 200 miles to fill water (electrolyte). It claims to have developed technology of using nano-silver filters that prevent carbon dioxide from entering the system, which used to be a problem earlier resulting in carbonization of the anode. Each anodic plate holds energy to carry an EV for 20 miles and the system currently holds 50 of the plates at one time, which add up to a capacity of 1000 miles. Once the anodes are depleted they must be replaced.
Zinc Air Batteries
Zinc has emerged as a candidate for energy source for EVs. Zinc–air batteries have some properties of fuel cells as well as batteries: the zinc is the fuel, the reaction rate can be controlled by varying the air flow, and oxidized zinc/electrolyte paste can be replaced.
Zinc–air batteries and zinc–air fuel cells are powered by oxidizing zinc with oxygen from the air. They have high energy densities and are relatively inexpensive to produce to the size needed for EVs propulsion.
The first rechargeable zinc-air batteries were produced in 1996 to power vehicles using AC-based drive-trains. The vehicles that first used zinc-air batteries were small and mid-sized buses in Singapore. The advantage of using the zinc–air batteries for EV propulsion is that earth’s supply of zinc is much more abundant than lithium. Research is on to see that zinc-air technology becomes commercialized.
Flow Batteries
A flow battery based on redox (reduction–oxidation), is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids contained within the system and separated by a membrane. Ion exchange occurs through the membrane while both liquids circulate in their own respective space. A flow battery may be used like a fuel cell or a rechargeable battery.
Recent research excitement to make flow batteries commercially viable is driven by issues around charging infrastructure of current LIBs. Such batteries have technical advantages over conventional rechargeable ones.
In flow batteries, separate liquid tanks (for anolyte and catholyte) can be mounted on the car itself (just like current gasoline tanks) and thereby unlimited longevity can be achieved. Electrolyte stored in this manner is usually pumped through the cells of the battery. They can be rapidly “recharged” by replacing the electrolyte liquid (in a similar way to refilling fuel tanks of IC engines) while simultaneously recovering the spent material for re-energization.
Various types of flow batteries have been developed, including redox, hybrid and membrane less. The fundamental difference between conventional batteries and flow cells is that energy is stored not as the electrode material in conventional batteries but as the electrolyte in flow cells.
Membrane less flow battery approach at Purdue University
A flow battery technology developed by Purdue researchers claim an “instantly rechargeable” method that is safe, affordable and environmentally friendly, akin to refueling a car at a petrol station.
Essentially, the technology uses, redox reactions in immiscible-fluids in porous media to achieve membrane less flow battery.
They claim to be the first to remove membranes from flow batteries leading to reduction in costs and extension of battery life. Membrane fouling can limit the number of recharge cycles and is a known contributor to many battery fires. Their startup called ‘IFBattery’ is producing components that are safe enough to be kept in a home garage and are stable enough to meet major production and distribution requirements in a cost effective manner.
MIT approach to develop an air-breathing flow battery
An MIT team has developed another kind of flow battery that breathes air, and can store energy long-term for about a fifth of the cost of existing technologies.
Their design of rechargeable flow battery uses anolyte which is made up of sulfur dissolved in water and they complemented it with a catholyte that is equally abundant (an oxygenated liquid salt solution). It turned out to be sodium which is a charge carrier to go back and forth between the sulfur and air electrode. In search for low cost positive electrode that could be used with sulfur cathode, they found out to be oxygen (air). The smart element of this battery is the fact that the catholyte “breathes” in air in from outside while discharging, and exhales while recharging. By this mechanism, the battery creates negatively-charged hydroxide ions in the catholyte while inhaling, and while recharging that oxygen is released, creating hydrogen ions which then send electrons back into the anolyte. Interestingly, unlike humans, this battery inhales and exhales oxygen and not carbon dioxide. This creates a charge balance by taking oxygen in and out of the system.
They believe that this battery would cost far less to make and run than lithium-ion batteries, while retaining almost the same energy density. Once in use, they estimate a scaled-up version of their flow battery would cost between US$20 and $30 per kWh stored to run, compared to about $100 per kWh for other storage systems.
Conclusion
The future of battery technology is very exciting, across the world in many research labs, the scientists are looking exciting breakthroughs. However, in the near and medium terms most of the research is focused on improving the already-commercialized lithium-ion.
How the battery market transforms itself in the next two decades, one does not know yet. However, one thing is certain that through human endeavor some of these technologies reviewed above may lead the charge to a 100% renewable future.
No token or token has expired.
Deprecated: Function get_magic_quotes_gpc() is deprecated in /home1/silvege7/public_html/paradigmconsultant.com/wp-includes/formatting.php on line 4371
